- News
Nat Aging | Zhao Senlin,Yan Dongwang and Li Dawei’s Collaborative Research Team Reveals the Hidden Force In the Progression of Colorectal Cancer--Aging Intestinal Epithelial Cells Adjacent to the Cancer
Colorectal cancer is the most common malignant tumor of the digestive tract in China, and the complex and variable tumor microenvironment is a key factor limiting its clinical prognosis improvement. For a long time, research on the tumor microenvironment has focused on various components that infiltrate within tumor tissue, such as stromal cells and extracellular matrix, but there has been little attention paid to tissues adjacent to the cancer.
According to the article published in Nature Communications (Nat Commun. 2024) by Professor Yan Dongwang from Shanghai General Hospital Affiliated to Shanghai Jiao Tong University School of Medicine and Dr. Zhao Senlin’s team from Fudan University Shanghai Cancer Center previously, colorectal cancer cells can transport intact mitochondrial DNA to adjacent colon epithelial cells through exosomes, triggering the metabolic reprogramming of epithelial cells and driving malignant progression of the tumor, thus revealing the “hypocritical mask” of adjacent colon epithelial cells and expanding the concept and connotation of the tumor microenvironment.
On December 3, 2025, the latest research progress made by Yan and Zhao’s team was published in Nature Aging --Peritumoral colonic epithelial cell-derived GDF15 sustains colorectal cancer via regulation of glycolysis and histone lactylation. It is found that colorectal epithelial cells adjacent to cancer acquire an aging phenotype and cooperate with tumor cells across domains to form a metabolic epigenetic synergistic feedback loop, further reshaping the intestinal pro cancer niche, thus providing a key theoretical basis for the paradigm innovation of colorectal cancer diagnosis and treatment.
Research team first applied high-throughput secretion omics chips, multi-color immunohistochemistry and other technologies and discovered that colorectal epithelial cells adjacent to cancer exhibit aging characteristics and have a unique aging related secretion spectrum, among which the secretion changes of GDF15 are very significant. Combined with conditional gene knockout mice and other models, it has been confirmed that the binding of GDF15-GFRAL ligand receptor activates the JNK1-STAT3-ENO1 signaling axis in cancer cells, continuously driving the formation of a high lactate microenvironment, thereby maintaining rapid growth of cancer cells.
In addition, research team found that lactate stimulation of intestinal organoids can increase the secretion of GDF15. Clinical cohort analysis and series of experiments further revealed that histone H4K8 site lactylation is specifically enriched in the GDF15 promoter region and can directly regulate GDF15 transcriptional expression.
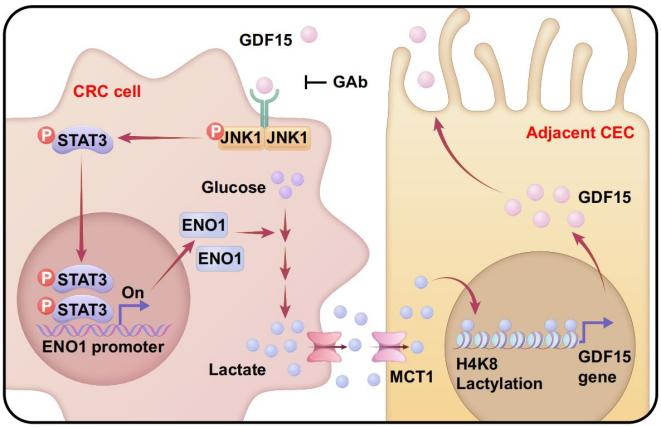
GDF15 and lactate signaling jointly construct a novel loop for communication between adjacent intestinal epithelial cells and tumor cells. This cross domain collaboration of metabolism and epigenetic modifications expands the understanding of the tumor microenvironment. This study suggests that clinical drug development based on adjacent cancer tissues may bring new hope for tumor treatment.
Dr. Zhao Senlin from the Department of Colorectal Surgery at Fudan University Shanghai Cancer Center, Professor Yan Dongwang from the Department of Colorectal Surgery at Shanghai General Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, and Dr. Li Dawei from Fudan University Affiliated Cancer Hospital are the co-corresponding authors of this article. The co-first authors of this article are Guan Bingjie, Zhou Mantang, Xie Bowen from Shanghai General Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Dai Weixing from Fudan University Shanghai Cancer Center, Dr. Zhang Jing from the Ninth People's Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, and Mi Yushuai from the Department of Gastroenterology at Shandong University Qilu Second Hospital. This research was also guided by Weiyingqi Cui of the Royal Swedish Institute of Technology, Chen Fei from the Cancer Research Institute of the Fudan University Shanghai Cancer Cente, Hu Ronggui of the Hangzhou Institute of Advanced Study and other scholars.
https://doi.org/10.1038/s43587-025-01023-9
