- News
Research Team Led by Professor WANG Honglin from Shanghai General Hospital Published an Article in Nature Aging, Revealing a New Intervention Strategy for Alzheimer's Disease
Alzheimer's Disease (AD) is one of the most common neurodegenerative diseases worldwide and a leading cause of cognitive impairment and death in elder adults. In addition to the classic pathology of amyloid beta plaques and tau protein tangles, neuroinflammation and its underlying metabolic abnormalities play a crucial role in disease progression. Microglia in the brain are important "guardians" in maintaining the homeostasis of the neural environment, but in Alzheimer's disease, they often become dysfunctional due to long-term activation.
On October 29, 2025, Wang Honglin's team published an innovative study in the internationally renowned academic journal Nature Aging, titled "Loss of MFE-2 infections microglial lipid homeostasis and drives neuroinflammation in Alzheimer's pathogenesis". The study found that lipid metabolism disorders in microglia are an important factor driving this process.
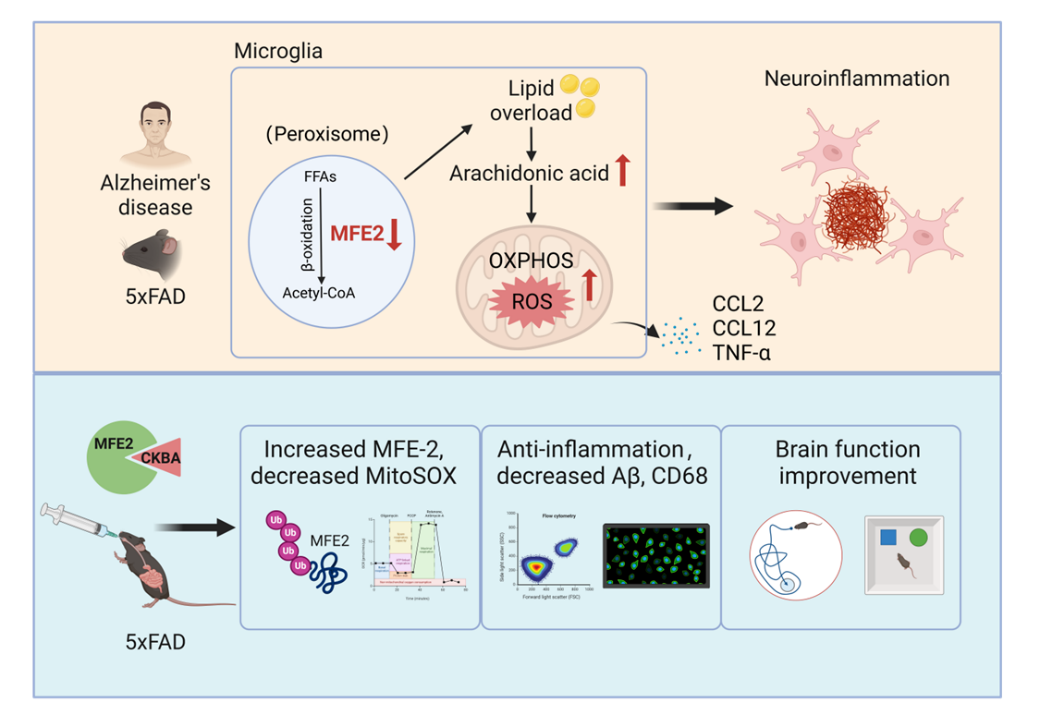
Research has shown that the peroxisome fatty acid β - oxidation key enzyme MFE-2 is significantly downregulated in Alzheimer's disease patients and model mice. Its absence leads to abnormal lipid accumulation, increased oxidative stress, and persistent inflammatory response, thereby exacerbating A β deposition and cognitive impairment. Further drug screening results showed that the compound 3-O-cyclohexanecarbonyl-11-keto - β - boswellic acid (CKBA) derived from natural products can bind to MFE-2 and restore its function, significantly improving neuroinflammation and pathological damage.
This study reveals for the first time the key targets of the metabolic inflammatory coupling pathway in early Alzheimer's disease, proposes a new strategy for targeted intervention of microglial lipid metabolism, and demonstrates the complete scientific research chain from mechanism research to drug discovery. The research team stated that this achievement provides new scientific basis and potential drug directions for the prevention and treatment of Alzheimer's disease, as well as useful insights for the modernization of natural product research. It proposes a new strategy for targeting microglial lipid metabolism to alleviate neuroinflammation, and also provides new ideas for the scientific application of natural product molecules in neurodegenerative disease intervention. The research team stated that they will continue to explore the pharmacological mechanisms and preclinical studies of CKBA in the future, contributing new theoretical basis and candidate drugs for the prevention and treatment of Alzheimer's disease.
Introduction to research team and funding projects
This study was conducted by Professor Wang Honglin's team at Shanghai General Hospital. The team has long been committed to the discovery of the pathogenesis and targets of autoimmune diseases, as well as drug development. In recent years, focusing on immune metabolism networks and small molecule intervention strategies, multiple original achievements have been made in the molecular design of natural product derived drugs, precise targeting mechanism analysis, and clinical translation research. The team has successively constructed multiple models of neurological diseases and developed a series of small molecule candidate drugs with blood-brain barrier permeability and neuroprotective effects. The research published in Nature Aging is an important achievement of the team in the full chain research layout of "basic mechanism target confirmation candidate drug animal validation transformation promotion".
This study is supported by the Original Exploration Program of the National Natural Science Foundation of China, the Experimental Animal Research Project of the Shanghai Science and Technology Innovation Action Plan, the National Natural Science Foundation of China, and the Chinese Postdoctoral Fund.
WANG Honglin
Research leader
Second-grade professor, doctoral supervisor, recipient of the National Science Foundation for Distinguished Young Scholars (2017), Chief Scientist of the Key R&D Program of the Ministry of Science and Technology (2020), currently serving as Deputy Director of the National Key Laboratory of Innovative Immunotherapy, Distinguished Professor at Shanghai Jiao Tong University, and mentor of the Zhiyuan Honor Program. He is the Executive Vice President of the Clinical Research Institute and Director of the Precision Research Center for Difficult Diseases at the First People's Hospital of Shanghai. Formerly served as the Deputy Director of Shanghai Institute of Immunology and was awarded the titles of "Oriental Scholar", "Pujiang Talent", and "Outstanding Discipline Leader of Shanghai Science and Technology Commission". I am currently the Vice Chairman of the Skin Immunology Branch of the Chinese Society of Immunology.
Wang Honglin has long been committed to the research of immune mechanisms and new therapeutic targets for chronic inflammation, and has achieved multiple innovative results. Published 78 papers in internationally authoritative journals, with a cumulative impact factor of 665 and over 5500 citations; Having 16 national invention patents and 4 PCT patents, it has significant academic influence and potential for industrial transformation.
GAO Min
First author
PhD, graduated from Shanghai Jiao Tong University School of Medicine in 2020 with a research-oriented doctoral degree in ophthalmology. The doctoral training unit is the Ophthalmology Department of Shanghai General Hospital. After graduating with a PhD, she conducted postdoctoral research at the Clinical Translational Research Institute of Shanghai General Hospital under the guidance of Professor Wang Honglin. During her postdoctoral stage, she received funding from the Shanghai "Super Postdoctoral" Incentive Program and the Postdoctoral Project. During her postdoctoral studies, Dr. Gao Min focused on the regulation and intervention of central inflammation mechanisms in Alzheimer's disease, delving into the regulation of microglial lipid metabolism and targeted intervention strategies. She is skilled in applying immunological techniques to analyze the inflammatory functional status of microglia and has a broad research perspective and outstanding innovation ability. She will continue to devote herself to transforming basic research achievements into clinically applicable treatment methods, promoting precise treatment and mechanism research of neurological diseases.
Article link: https://www.nature.com/articles/s43587-025-00976-1
