- News
Scientific Breakthrough | Professor Honglin Wang's Team at Shanghai General Hospital Reveals a Novel Neuro-Immune Pathogenic Mechanism and Therapeutic Strategy for Vitiligo in Immunity
https://mp.weixin.qq.com/s/GQFCZPScxawSiff8vC1qFQ

On June 16, the team led by Professor Honglin Wang from the Clinical Research Institute of Shanghai General Hospital published a research article in Immunity entitled:
“Nociceptor-derived CGRP enhances dermal type I conventional dendritic cell function to drive autoreactive CD8+ T cell responses in vitiligo.”
This groundbreaking study integrates single-cell sequencing, spatial transcriptomics, full-thickness skin clearing imaging, nearly ten types of genetically engineered mice, and an investigator-initiated clinical trial (IIT). It innovatively identifies a novel neuro-immune pathogenic mechanism in vitiligo involving the “sensory neuron–CGRP–cDC1–CD8+ T cell” axis.
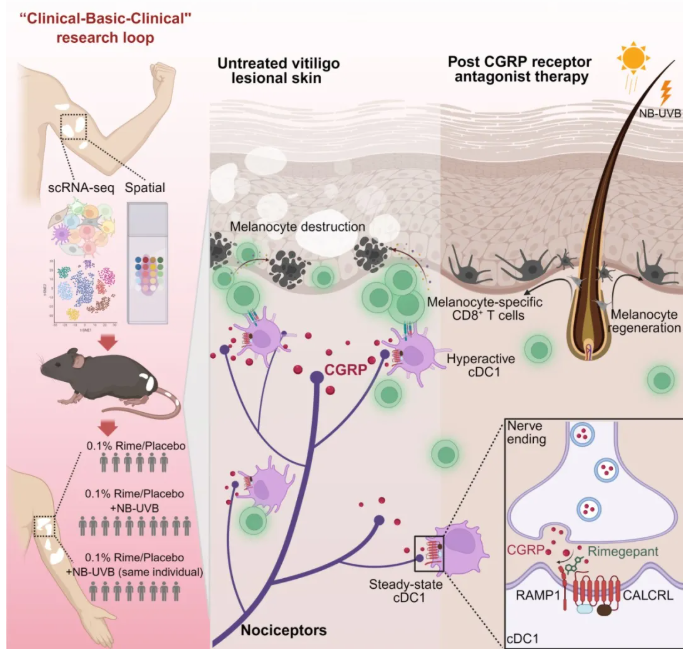
By targeting this axis with the CGRP receptor antagonist Rimegepant, researchers successfully inhibited disease progression in mouse models of vitiligo. Furthermore, the treatment demonstrated promising clinical efficacy in a trial involving 57 vitiligo patients, offering a new therapeutic strategy for this difficult-to-treat condition.
Vitiligo is a chronic, disfiguring autoimmune skin disease characterized by localized or widespread white patches due to melanocyte loss. In China, approximately 30 million people suffer from vitiligo, with over 50% developing the condition before age 20. The disease is lifelong and notoriously difficult to cure. Currently, the only FDA-approved drug for vitiligo is the JAK inhibitor ruxolitinib cream, which remains unavailable in China, leaving Chinese patients without any targeted therapy options.
To address this urgent clinical need, Professor Wang’s team at Shanghai General Hospital, in collaboration with Professor Aie Xu’s team from Hangzhou Third People’s Hospital, conducted original research demonstrating how sensory neurons in vitiligo lesions secrete CGRP, which activates dermal cDC1 cells. This activation significantly enhances their antigen presentation and co-stimulatory capabilities, triggering a robust CD8+ T cell autoimmune response against melanocytes. This leads to progressive destruction of melanocytes and white patch formation on the skin.
Blocking the CGRP–CALCRL signaling pathway using Rimegepant significantly inhibited cDC1 expansion and the cytotoxic CD8+ T cell response. This intervention helped create an immunosuppressive microenvironment conducive to melanocyte regeneration and migration under phototherapy, thereby offering a powerful new angle for vitiligo treatment.
This research not only addresses the critical shortage of targeted therapies for vitiligo but also uncovers a previously unknown “sensory neuron–CGRP–cDC1–CD8+ T cell” mechanism, offering a new theoretical framework and therapeutic target. The team’s newly developed 0.1% Rimegepant topical cream has shown encouraging clinical results, completing a full “clinic–lab–clinic” translational research cycle.
Doctoral students Xiuli Yang and Wenxiang Ding from Professor Wang’s team are co-first authors of the paper. This research was supported by:
The National Natural Science Foundation of China Original Exploration Program
National Natural Science Foundation Joint Fund Project
Shanghai Science and Technology Innovation Action Plan (Laboratory Animal Research Program)
Additional grants from NSFC and the Shanghai municipal government
In the field of translational medicine, Professor Wang’s team is dedicated to identifying novel targets and developing new drugs for stubborn dermatological diseases. Over two decades, they have modified and screened active compounds from the traditional Chinese medicine Frankincense, ultimately discovering the novel compound CKBA. This first-in-class molecule has secured patents in China, the U.S., Japan, and the EU, creating a robust global intellectual property portfolio.
The CKBA topical cream, a 1.1 class drug candidate with independent IP, is designed for the treatment of vitiligo and mild-to-moderate plaque psoriasis.
The Phase II clinical trial of CKBA cream is led by Professor Aie Xu, a leading vitiligo expert and director of the Institute of Dermatology at Hangzhou Third People’s Hospital. This multicenter, randomized, double-blind, vehicle-controlled, dose-finding study aims to evaluate the safety and efficacy of CKBA cream in non-segmental vitiligo patients.
Approved in July 2023, the trial completed the enrollment of 200 patients across 22 centers in China by October 2024. All participants completed the study by May 2025, and the team is now performing data cleaning and analysis. Upon unblinding of the data, an application for Breakthrough Therapy Designation will be submitted to the National Medical Products Administration (NMPA).
Original article link:
https://www.cell.com/immunity/abstract/S1074-7613(25)00239-0
Reporter: Clinical Research Institute
Editor: Cai Shishi, Communications and Spiritual Civilization Department
