- News
World’s First! Shanghai General Hospital Leads with the First Administration of Innovative Cell Therapy IGT001 for Retinitis Pigmentosa
https://mp.weixin.qq.com/s/2QA1JXtq4ALlOOeM6QBh2A
On May 16, 2025, the Department of Ophthalmology at Shanghai General Hospital—also home to the National Clinical Research Center for Eye Diseases—successfully completed the world’s first intravitreal injection of IGT001, an innovative cell therapy for a patient with Retinitis Pigmentosa (RP). The patient has since completed a 7-day postoperative follow-up, with stable systemic and ocular conditions, marking a critical first step in clinical research on this novel treatment for retinal degenerative diseases.
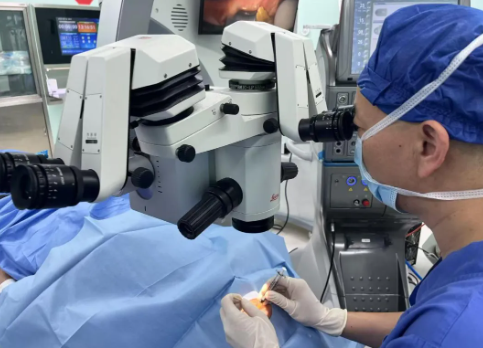
This clinical study, led by Professor Sun Xiaodong, Director of the Ophthalmology Center, is a first-in-human (FIH), investigator-initiated, prospective, open-label, non-randomized, dose-escalation trial. The primary objective is to assess the safety and tolerability of different doses of IGT001 administered via intravitreal injection in patients with RP. Additionally, the study aims to explore the potential efficacy of the therapy by monitoring changes in ocular function compared to baseline following a single injection.
Developed by InGel Therapeutics—a biotech company specializing in innovative ophthalmic treatments and founded on research from Harvard Medical School—IGT001 is the company’s first candidate therapy. Its core component is human rod photoreceptor precursor cells (hRP), delivered directly into the vitreous cavity. Designed as a broad-spectrum therapy, IGT001 is not limited to patients with specific genetic mutations, aiming to slow vision loss and, in some patients, enhance visual function.
The therapy features two key innovations: a unique cell-biomaterial co-delivery system that nourishes existing cone cells to help preserve and potentially restore their function, and a minimally invasive injection method with significant safety advantages.
Preclinical studies have shown that IGT001 significantly improves optomotor response (OMR) and electroretinogram (ERG) performance in rd10 mouse models of retinal degeneration. Histological analysis confirmed enhanced cone cell survival. The proprietary biomaterials used in IGT001 can be tailored to support different retinal cell types. For example, in treating retinal ganglion cells affected by glaucoma, the material has demonstrated protective effects. Findings have been published in leading journals such as Nature, underscoring the therapy’s potential to restore retinal function.

The success of this first-in-human administration marks a major milestone in the global development of IGT001, signifying a key breakthrough in its transition from laboratory research to clinical application. “The dose-escalation design of IGT001 balances safety with therapeutic exploration,” said Professor Sun. “Its broad applicability holds promise for RP patients with various genotypes.”
This landmark study is expected to generate critical data for advancing cell therapy in retinal degenerative diseases, bringing hope to patients worldwide.