- News
SGH 160th Anniversary |Xinmin Weekly features a report on Shanghai General Hospital: A Century-Old Institution at the Forefront of Scientific Research and Innovation
https://mp.weixin.qq.com/s/gjv3qB_8DtRzHsuOWaCfLA
In October 2024, Shanghai General Hospital (Shanghai General Hospital, affiliated with Shanghai Jiao Tong University School of Medicine), a prestigious Class A tertiary hospital with campuses in both Hongkou and Songjiang districts, spanning nearly 300,000 square meters and integrating medical care, rehabilitation, education, and research, celebrated its 160th anniversary. Over these 160 years of advancing through the waves of history, medical concepts and treatment technologies have evolved rapidly. Now, Shanghai General Hospital is poised to enter a transformative era powered by AI-enhanced diagnosis and treatment. Staying true to its longstanding mission of "serving the people," the hospital is actively pursuing six transformations and building six "high-standard bases," embodying the vision of a "hospital of the future." Through these efforts, Shanghai General Hospital is bringing cutting-edge medical technologies and management practices closer to patients, contributing to Shanghai’s goal of becoming a global medical center.
To commemorate the 160th anniversary of Shanghai General Hospital, the 38th issue of Xinmin Weekly in 2024 published a special series of articles celebrating the hospital's achievements. This edition features "A Century-Old Institution at the Forefront of Scientific Research and Innovation."

Recently, Shanghai General Hospital achieved a groundbreaking patent transfer agreement, valued at 600 million RMB in total, with an initial payment of 30 million RMB—a record-breaking milestone for Shanghai hospitals. Scientific research and innovation are essential for the high-quality development of Class A tertiary hospitals and are crucial to China’s medical technology innovation framework and the growth of the biopharmaceutical industry. Enhancing medical research and innovation capabilities is key to achieving the goals of a "Healthy China." At Shanghai General Hospital, clinical physicians and researchers are deeply committed to scientific innovation, focusing on solving diagnostic and treatment challenges encountered in clinical practice and exploring cutting-edge topics in disease prevention and treatment. As of 2023, the hospital has received 425 grants from the National Natural Science Foundation of China over the past five years, achieved the transformation of 30 patented results, and won 28 awards for scientific and technological progress, including the National Science and Technology Progress Award (Second Prize) and the Shanghai Science and Technology Progress Award (First Prize).
Notably, many of Shanghai General Hospital’s research achievements have swiftly translated into diagnostic and treatment techniques available to patients, offering new solutions for previously untreatable conditions.
In 2019, Shanghai General Hospital established the Clinical Research Institute.

Scientific achievements accelerate the transition from the laboratory to clinical practice. In early September 2024, the list of winners for the inaugural “Shanghai Municipal Hospital Clinical Innovation Award” was announced at the opening ceremony of the 2024 Public Hospital Governance Capability Modernization Construction Seminar at the Shanghai Shenkang Hospital Development Center. Ten high-quality clinical research achievements were recognized. Among them, the “Immune Age” in vitro diagnostic kit project led by Professor Wang Honglin’s team from Shanghai General Hospital was selected. After long-term exploration and breakthroughs, Professor Wang’s team derived inspiration from the progression of viral infections and proposed a new diagnostic concept of “immune age.” After over three years of in-depth research, the team cleverly developed an original diagnostic method for “immune age” based on systematic analysis and verification of bone marrow and peripheral blood samples. This method accurately assesses the body’s “immune age” with just 1 milliliter of sample, requiring only 1 hour and targeting a single major indicator. It provides a data-driven evaluation of the immune system's status, identifies potential “danger signals,” and supports disease early warning. This innovative “immune age” test method is not only simple and time-efficient but also relatively cost-effective. Therefore, this achievement has broad application prospects in health check-ups, age-related diseases, moderate to severe inflammation, cancer risk screening, and more. Currently, the patent for the “Immune Age” project and subsequent cooperative development have been transferred to a related biopharmaceutical company for a total contract value of 600 million yuan, with an initial payment of 30 million yuan, setting a record for the highest initial payment of patent transfers to medical institutions in Shanghai.
▲ In 2023, the “Immune Age” diagnostic research led by Professor Wang Honglin (second from right) successfully completed the transformation.

As a leading medical specialty in Shanghai, the ophthalmology department of Shanghai General Hospital has also accelerated innovation breakthroughs in recent years, achieving a series of translational results in the field of gene therapy. In 2021, the ophthalmology center of Shanghai General Hospital (National Clinical Medical Research Center for Ocular Diseases) initiated the first clinical research for gene therapy for “Congenital Leber’s Amaurosis” (LCA) in China. LCA is a hereditary retinal disease caused by mutations in the RPE65 gene. Data show that approximately 4,500 newborns in China are diagnosed with this rare hereditary eye disease each year. Once affected, patients may experience vision loss, reduced visual field, and frequent falls, severely impacting normal life. According to Professor Sun Xiaodong, Director of the Ophthalmology Center at Shanghai General Hospital, applying gene therapy technology to ophthalmic diseases is a perfect match: although the eyes are small, the ophthalmology field accounts for 24% of global clinical gene therapy trials, the highest proportion among all specialized fields. Three years ago, an 11-year-old girl named Xiao Yi (a pseudonym) from Henan joined the clinical study of gene therapy for LCA at the ophthalmology center of Shanghai General Hospital, becoming the first child in China to receive gene therapy for LCA. Today, Xiao Yi can attend school like other children, engage in outdoor activities at night, and has significantly improved her self-esteem. Helping more patients, who were previously destined to live in darkness, regain their vision through the latest gene therapy drugs is the most gratifying achievement for Professor Sun Xiaodong’s team. In 2023, the ophthalmology center developed a gene therapy drug for age-related macular degeneration, which has obtained clinical approval as a new drug and passed ethical review, with clinical Phase III trials soon to begin. To encourage more doctors to start scientific exploration and research based on clinical problems, Shanghai General Hospital established Shanghai’s first clinical research center in 2016 and created the Innovation Achievement Transformation Office. In recent years, the hospital has also built a performance assessment system based on clinical research. In addition to key clinical skills, patents and research results can also be used for professional title promotions and evaluations, further stimulating doctors’ enthusiasm for scientific innovation. According to Ma Lei, Deputy Director of the Hospital’s Research Department, in the past five years, the hospital has seen a surge in scientific and technological achievements, with a total of 30 medical research results successfully transformed, with a total contract value exceeding 600 million yuan.
Cross-disciplinary Collaboration Between Medicine and Engineering Sparks Innovation
In the collaboration between medicine and engineering, the seamless connection between clinical issues and industrial needs presents both challenges and opportunities. In the past, when biomedical companies wanted to conduct multi-center clinical research, the traditional approach was to have a leading PI (Principal Investigator) with industry influence head the project and search for sub-centers willing to conduct the clinical trials. However, this method often involved significant information gaps, making it feel like "opening a blind box." Hospitals were unaware of the company's leading projects, and companies were unsure which projects hospitals were interested in. Yan Biao, Director of the Research Department at Shanghai General Hospital, told reporters that in recent years, the hospital has taken proactive steps to move the process forward, collaborating closely with leading companies and top universities to build medical innovation alliances and create a core functional area for the development of the pharmaceutical industry.
On April 27, 2024, a breakthrough was made in the development and application of the domestically-produced "Magnetic Knife" system. Professor Wang Han, a leader in the radiology department at Shanghai General Hospital, collaborated with Professor Shen Guofeng and Professor Chen Yao's teams from the School of Biomedical Engineering at Shanghai Jiao Tong University, along with relevant companies, to successfully complete preclinical primate trials of the domestic "Magnetic Knife" system for treating essential tremor and tremor-dominant Parkinson's disease. This trial provided critical support for the "Magnetic Knife" system to enter human clinical trials and ultimately achieve domestication, marking a successful example of medical-engineering cross-disciplinary cooperation.
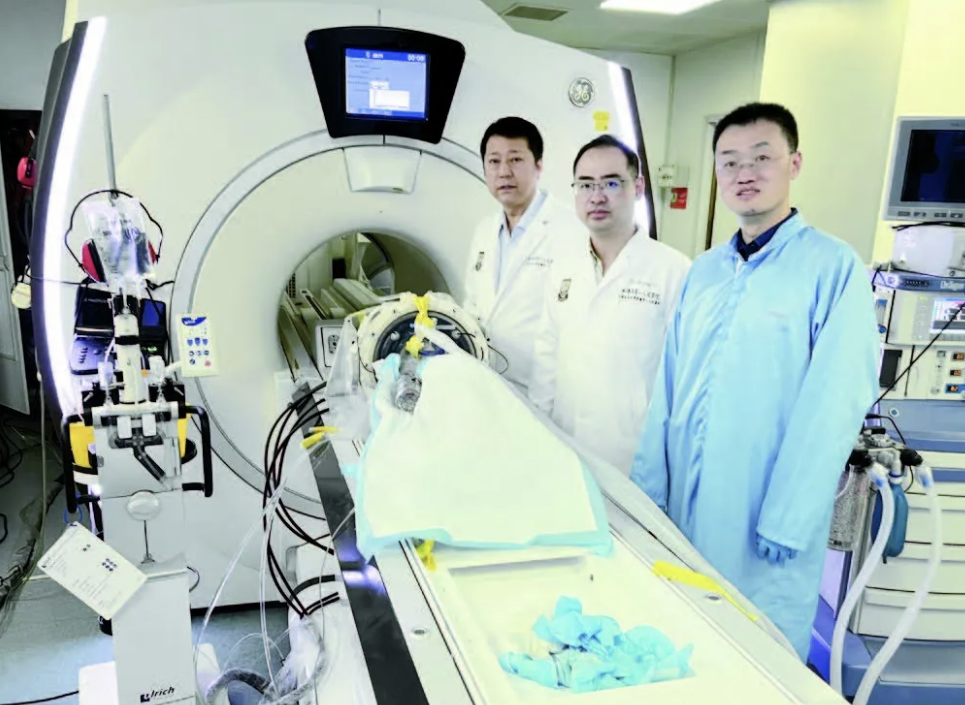
▲ In April 2024, Professor Wang Han's team successfully completed preclinical primate experiments on the domestic "Magnetic Knife."
The Bone Tumor Department at Shanghai General Hospital is one of the leading centers for bone tumor diagnosis and treatment in East China. In recent years, while providing high-quality medical services, the department has actively collaborated with medical enterprises to promote the clinical application of new biomaterials. "The five-year survival rate for malignant bone tumors has not significantly improved over the years, and clinical challenges like bone tumor metastasis, drug resistance, and precise excision and reconstruction urgently need to be addressed," said Cai Zhengdong, Head of the Orthopedic Center at Shanghai General Hospital and Director of the Shanghai Bone Tumor Research Institute. "Against this backdrop, there is an even greater demand for new technologies and drugs." At the end of 2021, the Bone Tumor Department led a project in collaboration with relevant companies, successfully undertaking the "14th Five-Year Plan" National Key Research and Development Program on "Diagnostic and Therapeutic Equipment and Biomedical Materials," focused on developing biomaterials to inhibit post-surgery recurrence of bone and skin tumors. In July this year, Director Cai Zhengdong led a multi-center registered clinical trial for the "absorbable artificial bone" material, which was officially approved by the National Medical Products Administration (NMPA). This product, using advanced preparation techniques, not only provides a favorable environment for bone cell growth but also significantly enhances the material's osteoinductive capabilities. Once implanted, the artificial bone gradually degrades and is replaced by autologous new bone, avoiding the need for a second surgery, reducing patient pain, and cutting medical costs.
Additionally, under the new "Industry-Academia-Research" model of medical-engineering cross-collaboration, the Prostate Disease Team of the Urology Department at Shanghai General Hospital worked closely with relevant companies to meet the clinical need for precise hemostasis and efficient cutting in prostate enlargement resection. They promoted innovation and transformation of thulium laser technology, completing several projects funded by the Shanghai Municipal Science and Technology Commission. Recently, both parties jointly developed a higher-performance 1.94μm wavelength thulium laser device using advanced optical fiber laser technology, significantly improving cutting precision and hemostatic effects. The intraoperative blood transfusion rate decreased from 2.0%-11.1% to 0%-1.3%, significantly reducing surgical complications. Through continuous technical iteration and upgrades, they collaborated with companies to bring the third-generation device to market and led the research and application of new clinical indications—treating urological tumors and stones, significantly driving the innovation, transformation, and application of domestic thulium laser treatment devices in China and providing more effective and safer new domestic devices for clinical diagnosis and treatment.
From these latest cases, it is clear that Shanghai General Hospital has built an innovative alliance that integrates medicine, research, and industry on a large scale, allowing for highly efficient collaboration and seamless conversion of research results into practical applications.
“Research Infrastructure” Supporting Innovation
For medical researchers, any innovation or development is hard to truly materialize without the support of laboratories and platforms. Gene therapy is a hot topic in the future breakthroughs of medicine, and Shanghai General Hospital has already laid the groundwork in this field. The medical "infrastructure" at the hospital, including disease databases, biobank, and research wards, has grown significantly, placing the hospital at the forefront of the industry. Currently, Shanghai General Hospital has successfully established a public platform that combines Good Clinical Practice (GCP) and Good Manufacturing Practice (GMP) in a "front clinic, back factory" model for gene therapy.
Gene therapy drugs are "living drugs," which require very strict storage conditions, including ultra-low temperatures. To ensure the drug’s activity, there is a strict time limit for reconstitution and dispensing at room temperature. The GMP pilot workshop is a specialized facility used to scale up laboratory research results and conduct preliminary industrial production. It serves as not just a transitional production site, but as an early key step in transforming laboratory products into usable medications, effectively saving time and transportation costs in new drug development and improving efficiency. The "front clinic, back factory" model facilitates clinical verification of preliminary safety and potential effectiveness directly within the hospital.
Furthermore, to address the reality that existing gene therapy technologies cannot effectively cover most common pathogenic mutations, Shanghai General Hospital has been building a disease database and biobank in recent years. This initiative aims to track the natural disease course of patients with common mutations in China. As early as 2011, the hospital began establishing the biobank. This is similar to a research effort where “the trees planted by previous generations provide shade for later generations,” and it requires time to accumulate results.
“Patients who visit general hospitals may require care from different departments at different stages of their illness, leaving behind valuable clinical resources,” says Chen Huan, Director of the Clinical Sample Resource Center at Shanghai General Hospital. “Therefore, the ‘biobank’ is also known as the Clinical Sample Resource Center, reflecting that it is both clinically derived and a valuable resource.”
The Clinical Sample Resource Center has partly solved the dilemma that clinicians face when starting research projects, where "a skilled woman cannot weave without silk." For instance, in cancer treatment, there is typically a 3-year and 5-year follow-up period after surgery, and doctors tend to focus on samples from those timepoints. However, samples from other time periods are also valuable, and this is where the biobank comes in.
In July 2024, the "Rare Disease Precision Cohort for Pediatric Osteosarcoma" was officially released, a joint initiative between the Department of Bone Tumors at Shanghai General Hospital and the Shanghai Bone Tumor Research Institute. This cohort spans over a decade and includes more than 300 clinical cases of osteosarcoma, along with multi-genomic data totaling over 30TB—making it one of the largest osteosarcoma cohorts in the world. This cohort has already been made available through the national shared platform to support clinical research and drug development for multiple research institutes and leading biopharmaceutical companies.
With the increasing internal demand for clinical research and innovation, clinical research wards have emerged at the hospital. Since 2016, under the leadership of the municipal government, the Shenkang Center has launched the “Clinical Innovation Three-Year Action Plan,” guiding municipal hospitals to transform into research-oriented and innovative institutions. In April 2018, the Phase I Clinical Trial Research Lab at Shanghai General Hospital was established, followed by the creation of the Clinical Research Institute in 2019 to further integrate the clinical research system and empower the development of new drugs and medical devices in the biopharmaceutical industry.
The research wards have seen a corresponding enhancement, and in 2023, the hospital received approval to build a city-level demonstration research ward. It currently occupies 1,300 square meters and includes 36 research beds. Ding Xueying, head of the Phase I Clinical Trial Research Lab, explained that the clinical innovation and transformation process previously faced challenges, such as the difficulty in integrating medical, industrial, and research sectors, low efficiency in the innovation transformation chain, and the difficulty in sustaining original and breakthrough results. However, the clinical research center and research wards are expected to improve these issues.
Since its launch, the research wards have focused on ensuring participant safety, while utilizing digital technologies and innovative management models to improve research management efficiency. Under the support of the research wards, the average startup time for clinical research projects has been reduced from 6 months to 1.5 months, recruitment time shortened by 6 to 12 months, and the number of ongoing projects has increased by 20% compared to the previous period. In 2024, the clinical research service contracts at Shanghai General Hospital are expected to exceed 100 million yuan.
In recent years, the research wards have led over 30 multi-center studies, publishing numerous high-quality clinical research papers and translating several high-level patents. Collaborations with basic science and epidemiological teams, such as the team led by Professor Peng Yongde at the Department of Endocrinology and Metabolism and Professor Zhao Liping’s team at the Shanghai Jiao Tong University National Key Laboratory of Microbial Metabolism, have resulted in publications in top journals like Science and Cell.
In pursuit of the overarching goal of building a research hospital with distinctive quality management, Shanghai General Hospital proactively established the Clinical Research Institute in December 2019. The institute houses four major centers: the National Clinical Research Center for Ocular Diseases, the Precision Research Center for Difficult Diseases, the Medical AI and Interdisciplinary R&D Center, and the Clinical Trials and Evidence-Based Research Center. Since its establishment, the institute has made significant progress in talent development. By the end of 2023, it had built 35 basic and clinical PI teams, with two members selected for the National Outstanding Youth Program, two for the National Young Talents Program, and six for the National High-Level Talent Program.
As a large, top-tier hospital, Shanghai General Hospital is committed to becoming a research-driven institution and a hub for biopharmaceutical and medical innovations, not only in China but also internationally. It aims to be the “last mile” of clinical research transformation, playing a key role in the development of innovative clinical medicine and industry-academia-research collaboration.
Source: Xinmin Weekly, Issue 38, 2024
Reporter: Wang Zhongyun
Editor: Publicity and Spiritual Civilization Department, Cai Shishi
Translator: International Cooperation and Exchange Department, Yuhan Wang
