- News
Led by the Bone Oncology Department of Shanghai General Hospital, the domestically developed new artificial bone has been approved for market launch
赋能医药产业,让骨缺损完美“重生”--市一骨肿瘤科牵头国产自主研发新型人工骨获批上市
Recently, the multicenter registered clinical trial "Absorbable Artificial Bone" material, led by Director Cai Zhengdong of the Orthopedic Oncology Department at Shanghai General Hospital, was officially approved by the National Medical Products Administration (NMPA). The registration certificate number is 20243131050.
Artificial bone substitutes are used in surgical procedures to fill bone defects caused by bone tumors or fractures and to induce new bone formation. They are an indispensable material in orthopedic surgeries. As high-value consumables, imported products are often expensive, while domestically produced options have so far been limited to simple inorganic compounds, such as calcium sulfate and tricalcium phosphate. This product, developed by Academician Xuesi Chen's team at Changchun SinoBiomaterials Co., Ltd., represents a new type of implantable biomaterial for orthopedic use. It features a unique composition of poly(lactic-co-glycolic acid) (PLGA) and hydroxyapatite (HA), making it the first organic-inorganic composite artificial bone material in China. This composite material overcomes the challenges of poor degradability in purely inorganic materials and suboptimal mechanical properties in organic materials. By mimicking the structure of human cancellous bone, the material combines the excellent mechanical properties of inorganic components with the osteoinductive characteristics of organic components. It effectively promotes autologous bone growth while being degradable in tandem with the bone formation process. Through advanced manufacturing techniques, this artificial bone provides an optimal environment for osteoblast growth and significantly enhances the material's osteoinductive capabilities. Once implanted, the artificial bone gradually degrades and is completely replaced by the patient's own new bone, eliminating the need for a second surgery to remove the implant. This reduces patient discomfort and overall medical costs. Furthermore, the excellent biocompatibility of the product ensures safety during the implantation process, minimizing the risk of immune reactions or rejection.
As the first domestically-produced product of its kind, this artificial bone will be significantly less expensive than imported alternatives. This not only helps to alleviate the financial burden on the national health insurance system but also directly reduces the economic strain on patients and their families, allowing more patients to access high-quality orthopedic treatment.

Before the surgery: A tumor lesion was observed in the medullary cavity.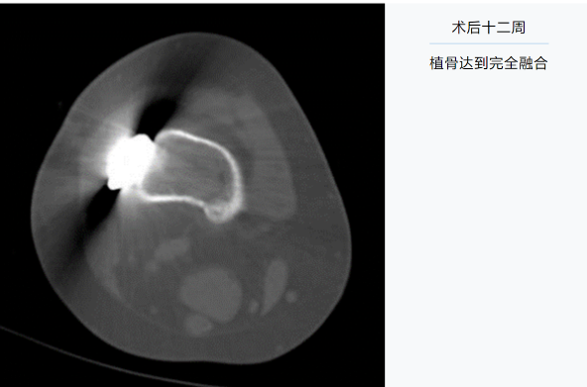
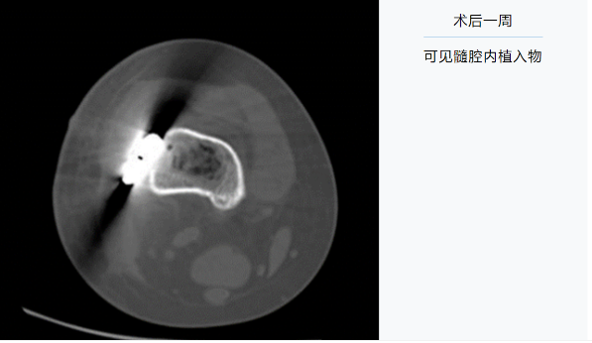
1 week after the surgery: Implants were observed in the medullary cavity.
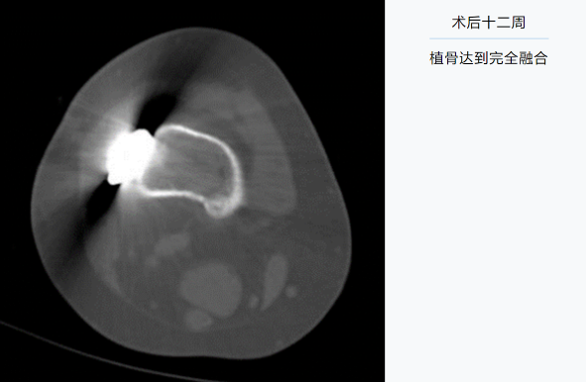
12 weeks after the surgery: the bone graft has achieved complete fusion.
Shanghai General Hospital's Department of Bone Tumors is one of the leading centers for bone tumor diagnosis and treatment in the East China region. The department not only provides high-quality medical services but also actively promotes the clinical application of new biomaterials. Through a long-term collaboration with Changchun Sinobio Biomaterials Co., Ltd., significant progress has been made in the field of medical polylactic acid derivatives.
At the end of 2021, the Department of Bone Tumors at Shanghai General Hospital, in conjunction with Changchun Sinobio Biomaterials Co., Ltd. and other institutions, successfully undertook a key project under the National Key Research and Development Program during the 14th Five-Year Plan. The project, titled "Development of Biomaterials to Prevent Recurrence of Bone and Skin Tumors After Surgery," focuses on developing biomaterials that can inhibit the recurrence of bone and skin tumors post-surgery.
Original link: “十四五”国家重点研发计划重点专项 “可抑制骨与皮肤肿瘤术后复发的生物材料研制” 项目启动会召开 (qq.com)
In September 2023, the Ministry of Industry and Information Technology and the National Medical Products Administration announced the first list of projects selected for the "Champion of Innovation" initiative in biomedical materials. The team from Shanghai General Hospital, together with Changchun Sbm Biomedical Materials Co., Ltd., was jointly selected for their outstanding achievements in the field of medical polylactic acid derivatives in polymer materials. This recognition highlights the team's significant contributions to the field.
Original link:上海市一骨肿瘤科荣获工信部揭榜挂帅联合单位 (qq.com)
The successful approval and market release of the new product further solidify the leading position of both parties in this domain and lay a solid foundation for future in-depth cooperation and innovative development.
Introduction to the Discipline
The Department of Bone Oncology at Shanghai General Hospital, founded by the renowned orthopedic and bone oncology expert Professor Cai Zhengdong, is one of the major centers in China for the diagnosis, treatment, research, rehabilitation, and prevention of bone and soft tissue tumors. The clinical specialties of the department include surgical resection and reconstruction for malignant bone tumors (in the limbs, pelvis/sacrum, and spine), limb-sparing treatment for pediatric malignant bone tumors, comprehensive/minimally invasive treatment for bone metastatic tumors, comprehensive treatment of advanced sarcomas, and clinical trials for new drugs. The department's case volume and clinical outcomes are at the forefront both nationally and internationally. Utilizing modern instrumentation, advanced comprehensive surgical treatment concepts, and compassionate care, the department serves a wide range of patients with bone tumors and bone diseases.
The Shanghai General Hospital, which has become a leading institution in its field after a decade of development, has focused its research on several critical areas: surgical resection and reconstruction techniques for bone tumors, new devices for limb-sparing surgery in children, the construction of a precision treatment system for bone tumors, and clinical studies of new treatment protocols for advanced sarcomas. The hospital has established a comprehensive research platform integrating cellular molecular studies, patient-derived xenografts (PDX), medical-engineering cross-disciplinary research, and clinical trials.
In the past five years, the institution has secured over 30 research grants, including those from national key research and development programs, the National Natural Science Foundation of China, and provincial and ministerial-level research funds. It has also received several prestigious awards and recognitions, including Shanghai Leading Talents, Shanghai Oriental Talent Top Talent Project, Shanghai Youth Top Talent, Shanghai Silver Snake Award, Shanghai Science and Technology "Qiming Star" Talent Plan, and Shanghai "Pujian Talent Plan."
The hospital's achievements in improving the efficacy of pelvic ring tumor resection and reconstruction have earned it the China Medical Science and Technology Award First Prize and the Shanghai Science and Technology Progress Award Second Prize. Research on the standardized surgery and precision treatment of osteosarcoma has been recognized with the Ministry of Education Science and Technology Progress Award First Prize and the Shanghai Science and Technology Progress Award Second Prize. These findings have been published in high-impact journals such as PNAS, Nature Communications, Advanced Science, and Oncogene.
Additionally, new targeted drugs for osteosarcoma, innovative surgical instruments for pelvic tumors, and liquid biopsy kits for bone tumors have been patented and received market approval, with further clinical trials underway. The hospital leads several domestic multi-center clinical studies, including clinical trials for TILs cell therapy in osteosarcoma, umbrella precision therapy for osteosarcoma, and oncolytic virus combined with TCR-T therapy for bone and soft tissue sarcomas, thereby enhancing patient prognosis and quality of life.
