- News
Traditional Recognition Subverted: Publication of Professor Yan Dongwang's Team From Shanghai General Hospital in a Nature Sub-Journal Indicates Peritumoral Normal Colonic Epithelial Cells Are Not “Normal”
https://mp.weixin.qq.com/s/LQcN7jhc4hf4oUvtLQFnZg
In China, the incidence of colorectal cancer ranks second among malignant tumors, second only to lung cancer, making it one of the most pressing medical challenges. As a major subtype of colorectal cancer, colon cancer is generally insensitive to immunotherapy, leading to poor patient prognosis. This suggests that abnormalities in the immune system within the tumor microenvironment are not the only factors promoting cancer.
In fact, throughout the development of colon cancer, it is surrounded by a large number of normal colonic epithelial cells. Although these epithelial cells are in close contact with cancer cells, they have traditionally been regarded as healthy cells and used as normal controls in medical research, severely overlooking their potential roles in promoting or inhibiting cancer.
Recently, the latest research by Professor Yan Dongwang’s team at Shanghai Jiao Tong University has timely filled this gap in understanding, innovatively correcting previous academic misconceptions. This work, titled “Mitochondrial genome transfer drives metabolic reprogramming in adjacent colonic epithelial cells promoting TGFβ1-mediated tumor progression,” was published in the prestigious international journal Nature Communications. Professor Yan Dongwang from the Department of Colorectal Surgery at Shanghai General Hospital is the corresponding author. Guan Bingjie, a direct Ph.D. student from Shanghai Jiao Tong University School of Medicine (2019 cohort), along with Dr. Liu Youdong and Dr. Xie Bowen from Shanghai General Hospital, and Dr. Zhao Senlin from Fudan University Shanghai Cancer Center, are co-first authors. Shanghai General Hospital is the first author affiliation. This research received valuable support and assistance from Researcher Wang Yiping of Shanghai General Hospital.

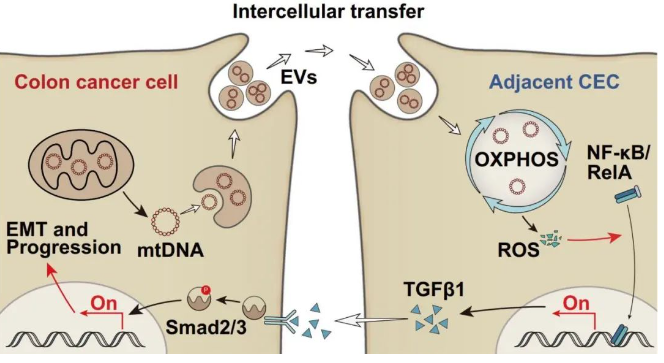
This innovative achievement challenges traditional tumor concepts and academic stereotypes, discovering and confirming for the first time that the normal colonic epithelial cells adjacent to colon cancer are, in fact, not “normal.” Instead, they collaborate with cancer cells to form a pro-tumorigenic “soil.” Specifically, normal colonic epithelial cells uptake intact mitochondrial genomes (mtDNA) from colon cancer cells, enhancing mtDNA-encoded protein expression, increasing mitochondrial complex activity, accelerating mitochondrial oxidative phosphorylation, and elevating reactive oxygen species (ROS) levels. The excessive ROS in normal colonic epithelial cells drive the transcription factor RelA into the nucleus, promoting TGFβ1 transcription and paracrine feedback that facilitates epithelial-mesenchymal transition in cancer cells, ultimately leading to colon cancer progression.
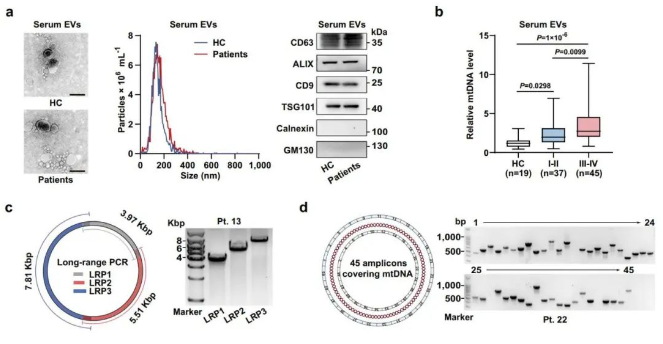
Researchers first discovered that the mtDNA content in serum extracellular vesicles of colon cancer patients was abnormally elevated and closely related to tumor staging. Using long-range PCR and full mitochondrial genome multi-fragment amplification techniques, they demonstrated that extracellular vesicles in patients' serum and secreted by colon cancer cells were rich in mtDNA, which maintained a complete circular structure. Additionally, the research team found that oxidative metabolic indicators in the mitochondria of adjacent normal colonic epithelial cells were significantly enhanced in patient samples and mouse models of in situ colon cancer. Based on this, they hypothesized that mitochondrial metabolism in colonic epithelial cells might be regulated by mtDNA in tumor-derived extracellular vesicles.
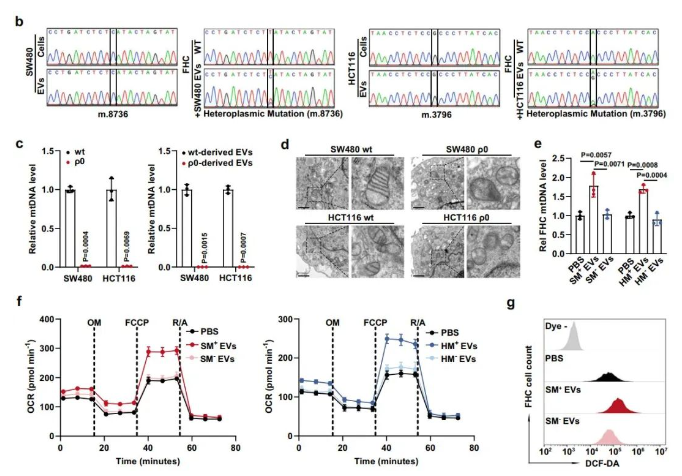
Subsequently, researchers confirmed that tumor-derived extracellular vesicles could indeed upregulate oxidative phosphorylation levels in adjacent epithelial cells while supplementing exogenous mtDNA. Given that mtDNA needs to enter mitochondria to exert direct regulatory functions, the team employed laser confocal microscopy, heterozygous point mutation sequencing, cross-species cell co-culture, and mitochondrial transfer experiments to clarify that tumor-derived extracellular vesicles transport mtDNA (rather than mitochondrial particles) into the mitochondria of adjacent epithelial cells.
To clearly demonstrate that exogenous mtDNA is crucial for enhanced oxidative phosphorylation in adjacent epithelial cells, researchers constructed mtDNA-knockout tumor cell lines and their extracellular vesicles. Through a series of in vitro cell, organoid, and in situ colon cancer mouse model experiments, they confirmed that mtDNA transfer mediated by tumor-derived extracellular vesicles is the main driving force behind abnormal mitochondrial oxidative metabolism in adjacent colonic epithelial cells.
At this point, an unexpected discovery intrigued the researchers: in colon cancer mice, the transfer of mtDNA by tumor-derived extracellular vesicles accelerated tumor growth. This strongly suggested that metabolically reprogrammed adjacent epithelial cells might, in turn, promote tumor progression. Cell co-culture experiments supported this novel phenomenon. The research team further elucidated this by employing transcriptome sequencing, cellular and animal experiments, and clinical tissue multicolor immunofluorescence staining, indicating that intercellular mtDNA transfer induces adjacent epithelial cells to produce large amounts of pro-tumor cytokine TGFβ1, which positively feedbacks to promote epithelial-mesenchymal transition and malignancy in colon cancer cells.
In-depth mechanistic exploration revealed that mtDNA transfer causes ROS accumulation in adjacent epithelial cells, which forces the transcription factor RelA into the nucleus to transcriptionally activate TGFβ1. Bioinformatics analysis combined with dual-luciferase reporter gene experiments and chromatin immunoprecipitation techniques identified the specific interaction sites where RelA drives TGFβ1 transcription.
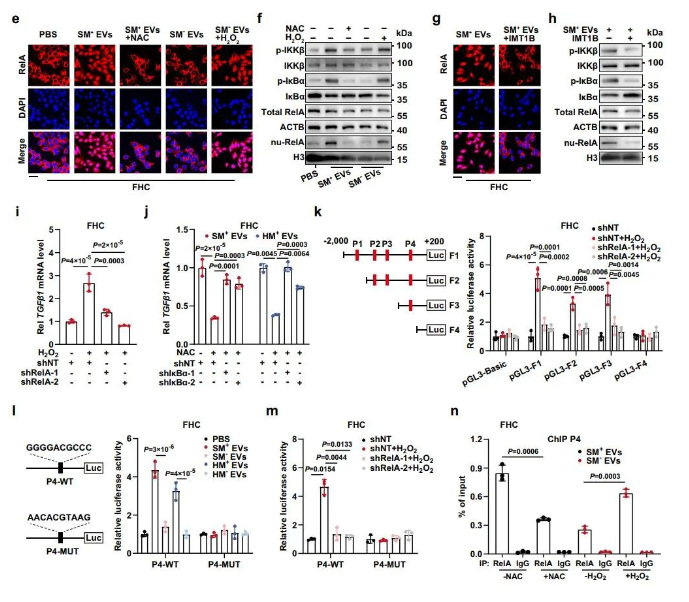
This research team is the first to propose the existence of a crucial metabolic communication loop between colon cancer cells and adjacent colonic epithelial cells, unmasking the hypocritical nature of adjacent colonic epithelial cells and revealing them as new “culprits” in cancer promotion. Its significance lies in opening new horizons for metabolic research in the tumor microenvironment and paving new paths for breakthroughs in colorectal cancer diagnosis and treatment.
The research team stated that intricate connections likely exist between adjacent colonic epithelial cells and cancer cells, and their findings indicate that this is just the tip of the iceberg. Beyond mtDNA, what other core molecules mediate this interaction? How can these foundational findings be translated into clinical practice? These are directions and hopes for future research.
