- News
The Shanghai Bone Tumor Institution of Shanghai General Hospital and the National Research Center for Translational Medicine (Shanghai) of Ruijin Hospital Jointly revealed the Molecular Subtypes of Osteosarcoma and Cracked the Key Mechanism of the "Bottleneck" in the Prognosis of Osteosarcoma
Osteosarcoma is the most common primary malignant bone tumor, which is prone to occur in children and adolescents. It features high invasion and high metastasis, and seriously threatens the life and health of children and adolescents. In the past 30 years, Drug treatment of osteosarcoma has been slowly progressed, and the five-year survival rate has been maintained in the "platform period" for a long time. The main reason is that osteosarcoma is highly heterogeneous and its genetic background is complex. Previous studies have mainly focused on the revelation of osteosarcoma mutation information, and lack of detailed description of osteosarcoma genetic panorama based on multi-dimensional data, so the genetic background of osteosarcoma is still not very clear.

The research progress of osteosarcoma genomics has been explored for ten years, and individualized and precise treatment has emerged.
Recently, the Shanghai Bone Tumor Institution of Shanghai General Hospital and the National Research Center for Translational Medicine (Shanghai) of Ruijin Hospital jointly published a research paper on osteosarcoma multiomics. Through the study of genome, transcriptome and epigenetics of tumor tissue from patients with osteosarcoma in Shanghai General Hospital (SGH-OS cohort), researchers systematically completed the panoramic analysis of gene structure and gene regulatory variation in the process of osteosarcoma occurrence and development, and established a new strategy for precise molecular typing and treatment intervention of osteosarcoma based on multi-omics data. The relevant research results were published in Nature Communications on November 23, 2022.
As the first large-scale genetic cohort of osteosarcoma, this study identified that genomic instability caused by gene mutations related to homologous recombination deletion and mismatch repair is the most prominent feature of osteosarcoma. By integrating the genome copy number variation, chromatin DNA methylation and gene expression profile, this study revealed that osteosarcoma can be classified into four molecular subtypes, namely, immuno-activated type, immunosuppressive type, homologous recombination defect type and MYC-driven type. The carcinogenic mechanism of various subtypes of osteosarcoma was systematically explored. Among them, the patients with immune activation subtype are characterized by strong immune response and the best prognosis; Immunosuppressive patients show IFN- γ Mediated immunosuppression, patients with this subtype may be the real beneficiaries of immunocheckpoint inhibitors. Homologous recombination-deficient subtype osteosarcoma showed high expression of cell cycle-related genes and the highest genomic instability. The corresponding intervention strategies were first-line platinum chemotherapy and PARP inhibitors. MYC-driven osteosarcoma is characterized by the amplification of the genome MYC gene and the high expression of the MYC gene. This subtype of osteosarcoma has the worst prognosis and the five-year survival rate is less than 40%. The improvement of the prognosis of this subtype of patients may be the key link to overcome the "bottleneck" of the overall prognosis of osteosarcoma.
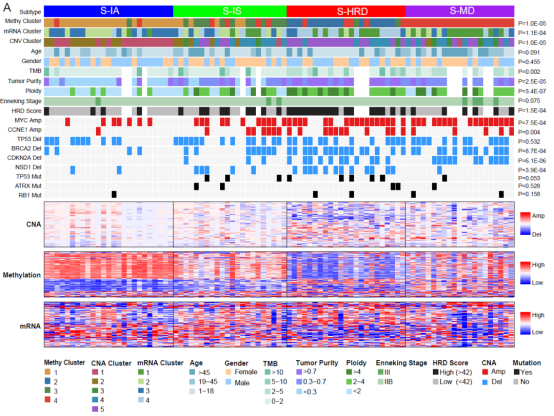
This study thoroughly analyzes the genetic background of osteosarcoma, explores the carcinogenic driving factors and new intervention strategies of different molecular subtypes of osteosarcoma, which will help to further identify the prognostic related biomarkers and chemosensitivity prediction biomarkers in osteosarcoma, and lay a foundation for the development of corresponding targeted treatment strategies. As the paper review experts said: "This is the most impressive and systematic genome study of the osteosarcoma large cohort so far" , "This multi-omics study based on the osteosarcoma large cohort provides a comprehensive variation map of the osteosarcoma genome, epigenome and transgenome. The comprehensive analysis method of multi-omics has well overcome the analysis bias caused by the significant heterogeneity of osteosarcoma".
Professor Hua Yingqi and Professor Cai Zhengdong of the Shanghai Bone Tumor Institute, the Department of Orthopedic Oncology, Shanghai General Hospital affiliated to Shanghai Jiaotong University School of Medicine, and researcher Wang Shengyue of the National Research Center for Translational Medicine (Shanghai) of Ruijin Hospital affiliated to Shanghai Jiaotong University School of Medicine, are the corresponding authors of the paper, and Dr. Jiang Yafei from Shanghai General Hospital affiliated to Shanghai Jiaotong University School of Medicine, Dr. Wang Jinzeng, graduate student fromthe the National Research Center for Translational Medicine (Shanghai) of Ruijin Hospital affiliated to Shanghai Jiaotong University School of Medicine, and Dr. Sun Mengxiong from Shanghai General Hospital affiliated to Shanghai Jiaotong University School of Medicine, are the co-first authors of the paper. This study was supported by the National Key Research and Development Plan of the 14th Five-Year Plan, the National Natural Science Foundation, the major clinical research project of the Shenkang Hospital Development Center, and the project of the National Major Science and Technology Infrastructure (Shanghai) Project of Translational Medicine.
