- News
Nature Metabolism: Professor Qinghua Zhao/Professor Ming He from Shanghai Jiao Tong University Collaborated to Reveal A New Mechanism of Liver/Bone Axis Steady State Regulation in Anti Osteoporosis
Link of the original article:
https://www.nature.com/articles/s42255-023-00803-0
The mechanism of liver/bone axis homeostasis regulation in anti osteoporosis has been newly discovered.
Recently, a research team led by Professor Qinghua Zhao from the Clinical Medical Center of Orthopedics, Shanghai General Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, and Professor Ming He from the Department of Pathophysiology, Shanghai Jiao Tong University School of Basic Medicine, published the latest research result in the journal Nature Metabolism: SIRT2 regulations extracted vessel mediated live one communication.
Osteoporosis is a common systemic metabolic bone disease. The destruction of the delicate balance between osteoclast driven bone absorption and osteoblast mediated bone formation is the pathogenesis of osteoporosis. In addition to the close cooperation between bone cells, bone metabolism is also regulated by complex communication between other organs, providing new insights for the treatment of osteoporosis. The liver is an important organ that regulates many physiological and pathological processes, including systemic glucose, lipid, and vitamin D metabolism. Chronic liver disease patients often have bone metabolism abnormalities, with about 75% of chronic liver disease patients suffering from severe osteoporosis, indicating that the liver plays a crucial role in regulating bone metabolism. However, the interaction between liver and bone metabolism is still largely unknown. There are few reports on the role of the liver in primary osteoporosis. Sirtuin2 (SIRT2) is a nicotinamide adenine dinucleotide (NAD+) dependent deacetylase that plays an important role in regulating life activities such as aging, metabolism, inflammation, and tumor development. The effect of SIRT2 in liver cells on bone homeostasis is still unclear.
The team discovered a new mechanism of liver bone communication regulated by liver cell SIRT2 in the study of elderly osteoporosis. The expression of SIRT2 in liver cells of elderly mice and elderly people increased; Liver specific SIRT2 deletion can inhibit the generation of osteoclast and alleviate bone loss in elderly osteoporosis mice. Further use of gene knockout mice, Protein mass spectrometry, RNA sequencing and other technologies to clarify the mechanism: leucine rich α- 2-Glycoprotein 1 (LRG1) is a functional protein in small extracellular vesicles (sEVs) derived from liver cells. In SIRT2 deficient liver cells, LRG1 levels in sEVs are upregulated, and LRG1 metastasis to bone marrow mononuclear cells (BMDMs) increases, thereby reducing NF- κ The nuclear translocation of Bp65 inhibits the differentiation of osteoclast. Treatment with sEVs carrying high levels of LRG1 can inhibit the differentiation of osteoclast in human BMDMs and osteoporosis mice. Meanwhile, by exploring the correlation between bone metabolism related parameters and plasma sEVs LRG1 levels, the research team found that plasma sEVs LRG1 was positively correlated with patients' BMD and negatively correlated with bone resorption markers. Therefore, this study suggests that drugs targeting hepatocyte osteoclast communication may be a potential strategy for the treatment of primary osteoporosis in the future.
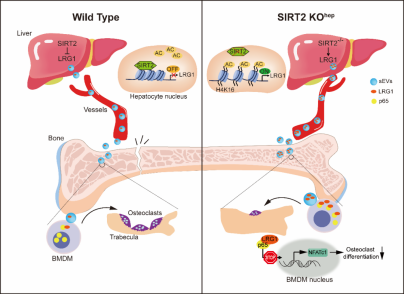
Mechanism diagram: New mechanism of liver bone communication regulated by SIRT2 in liver cells.
In general, this study explains the specific mechanism of hepatocyte osteoclast communication, and proves that the liver plays a key role in regulating osteoclast differentiation. SIRT2 KOhep upregulates the transfer of liver LRG1 to BMDMs through sEVs derived from liver cells, thereby inhibiting NF- κ B p65-NFATc1 activation and osteoclast production. Based on this, it is expected to develop new effective therapies for treating osteoporosis.
Professor Qinghua Zhao, Clinical Medical Center of Orthopaedics, Shanghai General Hospital Affiliated to Shanghai Jiaotong University School of Medicine, and Professor He Ming, Department of Pathophysiology,Shanghai Jiaotong University School of Basic Medicine, are the corresponding author of the paper. Professor Qinghua Zhao, graduate student Lin Longshuai, is the lead author of the paper. The research work received assistance and support from Academician Chen Guoqiang, Professor Zheng Junke, Professor Hong Dengli, Professor Zhao Qian, and Professor Long Xidai of Youjiang Ethnic Medicine College from Shanghai Jiao Tong University School of Medicine.
